Novocure’s Second Generation Optune System for Glioblastoma Now FDA Approved
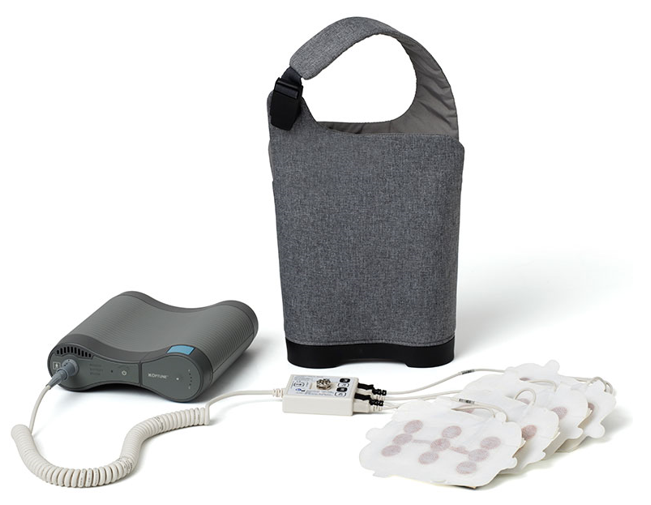
Novocure, a company now headquartered on the Jersey Isle, has announced FDA approval of the second generation of its groundbreaking Optune system. The Optune delivers so-called Tumor Treating Fields (TTFields) that interfere with cell division, in a sense pausing the development of tumors that are otherwise extremely difficult to treat. When cells divide they create mitotic spindles, tiny strings that pull on chromosomes to pry them apart for duplication. These spindles are susceptible to electric fields due to a natural charge, so finely tuning the field can prevent their activity and, if accurately directed, stop tumors from growing.
The system has been shown to be pretty effective, while exhibiting few side effects when compared to chemo. The power system of the new version of Optune is less than half the weight and size of the original, weighing in at 2.7 pounds (1.2 Kg), which should be a lot more convenient while you hunt for Pokémons on the go. The system also looks a lot more modern, less like a medical device and more like a portable audio system, and it even detects the light levels around it, adjusting the brightness of the indicators on the charger and device.
There is a battery indicator and built-in alerts that will warn when power reserves are running low. When ready to re-energize, the new Optune has been made hot-swappable, enabling patients to change the batteries or connect and disconnect from power without stopping the delivery of the electric fields into the brain.
The company intends to have all new patients receive the new generation of the system, while transferring all the existing patients to it in the coming weeks.
http://www.medgadget.com/2016/07/novocures-second-generation-optune-system-glioblastoma-now-fda-approved.html